Breakthrough drug in the treatment of Huntington’s Disease!
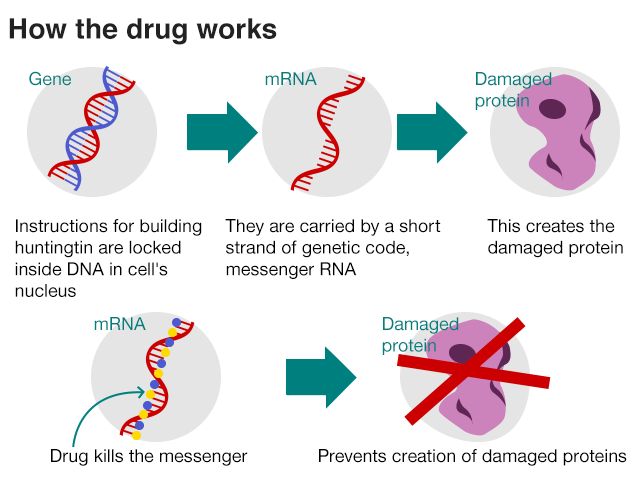
Huntington’s disease (HD) is a devastating neurodegenerative disease. Patient’s brain cells are in decline and this leads symptoms such as: memory loss, mood changes, jerky movements and results in death. The cause of this terrible disease comes from an error in the Huntington’s gene, a CAG DNA repeat that is much longer in HD patients than in people without the disease. This fault is carried in the messenger RNA, which in turn leads to the creation of a faulty Huntington’s protein. Source: http://www.bbc.com/news/health-42308341
The trial included 46 patients who were injected with the drug, in the spinal fluid. Researchers then looked at whether the drug was safe and whether it lowered the amount of faulty protein in the brain. Their findings follow on from the mouse study where they found that by lowering the amount of mutant Huntington’s protein motor functions recovered.
The drug now needs to go through phase 3 clinical studies, which will further establish the safety of the drug and also look at the effects on the drug, on the symptoms of Huntington’s disease such as motor functions.
This has the potential to open the doors for treatments into other neurodegenerative diseases such as Parkinson’s and Alzheimer’s.

Definitions
DNA is an acid in the chromosomes in the centre of the cells of living things. DNA determines the particular structure and functions of every cell and is responsible for characteristics being passed on from parents to their children. DNA is an abbreviation for 'deoxyribonucleic acid'.
RNA is an acid in the chromosomes of the cells of living things which plays an important part in passing information about protein structure between different cells. RNA is an abbreviation for 'ribonucleic acid'.
CAG repeat diseases: A group of neurodegenerative diseases characterised by the repetition of the nucleotides cytosine-adenine-guanine in-specific genes. Diseases in this group include Huntington’s disease.
For more see:
HD Research Update
August2018
Anotherstep forward in the continuing fight to defeat this catastrophic disease

IONIS-HTTRx (RG6042) Granted PRIME Designation by the European Medicines Agency for the Treatmentof People with Huntington's disease. IONIS-HTTRx is the first and only drug to demonstrate reduction of mutant huntingtinprotein, the underlying cause of Huntington's disease, in patients. Roche plans to initiate a pivotal study ofIONIS-HTT Rx (RG6042).
PRIME (PRIority Medicines) designationallows the drugs testing timeline to be sped up and the EMA will prioritiseassessment of the drug. Thisdesignation is usually given to drugs for diseases such as HD, where there areno available treatments.
"PRIMEdesignation for IONIS-HTTRx accelerates the review timelines and enhancesinteractions with the EMA, which can bring this potentially disease-modifyingdrug for people with Huntington's disease to regulatory approval faster. Thisdesignation will be useful as we work closely with Roche to quickly advanceIONIS-HTTRx into a pivotal study," said Dr. C. Frank Bennett, senior vicepresident of research and franchise leader for the neurological programs atIonis Pharmaceuticals. "This is our second antisense drug to demonstrate astrong safety profile and significant target engagement in the human centralnervous system. This profile gives us further confidence in the potential ofthe many other drugs we have advancing in R&D for the treatment ofneurological diseases."
TheFDA has also granted IONIS-HTTRx orphan drug designation. This is the USequivalent of the PRIME designation and again is for drugs that are treatingdiseases where there is no treatment.
ABOUTIONIS-HTTRx (RG6042)
“IONIS-HTTRx(RG6042) is an antisense drug designed to reduce the production of all forms ofthe huntingtin protein (HTT).” In particular it targets the mutant protein form(mHTT), accumulation of which leads to HD.
“In a Phase 1/2 study, IONIS-HTTRx (RG6042) demonstrateda significant reduction in mHTT, which breaks down the nerve cells in thebrain. The study demonstrated a mean 40% (up to 60%) reduction of the specificHD protein in the cerebrospinal fluid (CSF) of adult patients treated withIONIS-HTTRx (RG6042) for three months at the two highest doses. Furthermore,levels of mHTT measured in the CSF were still declining in the majority oftreated patients (~70%) as of the last measurement in the study. IONIS-HTTRx (RG6042) was well tolerated in thisstudy.”
Phase 3 will involve long termstudies which will look at patient safety and side effects in a larger patientpopulation. For more information: